Our Mission
Our Patients
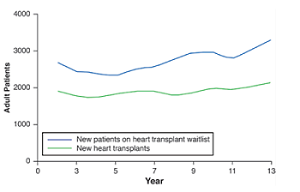
As the leading cause of death in the U.S., cardiovascular diseases affect millions of individuals each year. As seen in the figure, there are an increasing amount of heart failure patients while the number of potential heart donors have stayed constant, making the heart transplants, the gold standard for heart failure, a limited resource [1]. Over the last decade, left ventricular assist devices (LVAD) have emerged as an alternative treatment option in patients with advanced heart failure. However, despite significant improvements in survival and functionality, LVAD complications exist and can be difficult to predict.The most common complications include infections, followed by ischaemic neurological events, pump thrombosis, and bleeding. To help prevent these issues, CircuiTech has envisioned a more reliable LVAD; through wireless power transfer capabilities, LVADs have the possibility to be safer and more efficient than the models used today.
Competitor Analysis
Our biggest competitors in this sector are Leviticus Cardio, Thoratec, Heartware and ReliantHeart. All of these companies are working to create a wirelessly powered left-ventricular assist device. Recently, Leviticus Cardio did have success in implanting a device they have dubbed a “fully implantable ventricular assist device (FIVAD)” into a patient [2]. Their device lasts for 8 hours before needing a recharge and contains a back-up system to allow for traditional wired power in the event of a wireless failure. However, the device’s bulky nature and needed improvement in power transfer efficiency allows for Circuitech to grab hold of this market through our device.
Works Cited
[1] M. Colvin, J. M. Smith, M. A. Skeans, L. B. Edwards, K. Uccellini, J. J. Snyder, A. K. Israni, and B. L. Kasiske, “OPTN/SRTR 2015 Annual Data Report: Heart,” American Journal of Transplantation, vol. 17, pp. 286–356, 2017.
[2] Leviticus Cardio, "Leviticus Cardio COPLANAR ENERGY TRANSFER" [Online]. Available: http://leviticus-cardio.com/products.asp [Accessed: 01-May-2019].